Atoms and Electrons
When we look at the world around us, we see thousands of different objects. Some ‘objects’ are alive, some are organic, and many are inorganic.
There seem to be infinite ways in which matter can be composed.
The clothes we wear, the furniture we use, and the electronic devices that we rely on are all made of different types of materials. These materials have different properties, including chemical structure, density, optical, thermal, and electrical properties.
Yet every form of matter is made of only about 100 different elements, each representing a different kind of atom. Every kind of material that we know of uses the same ‘menu’ of atoms in different ways that make each material unique.
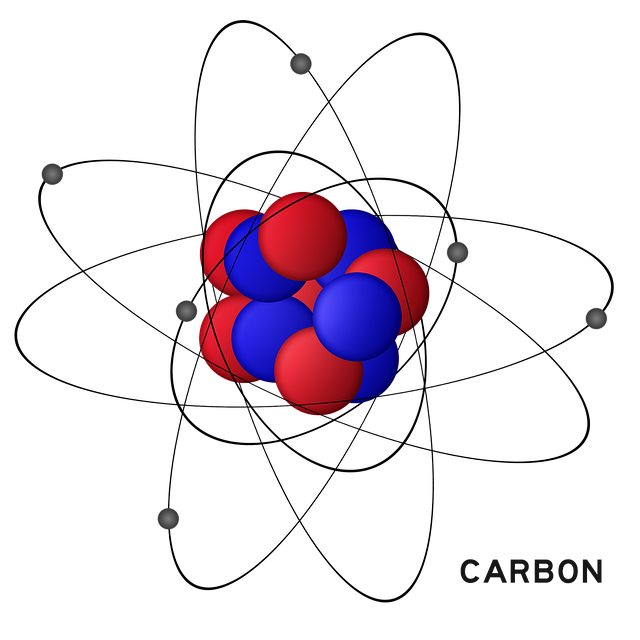
A carbon atom has 2 electrons in the inner shell, and four electrons in the valence (outer) shell.
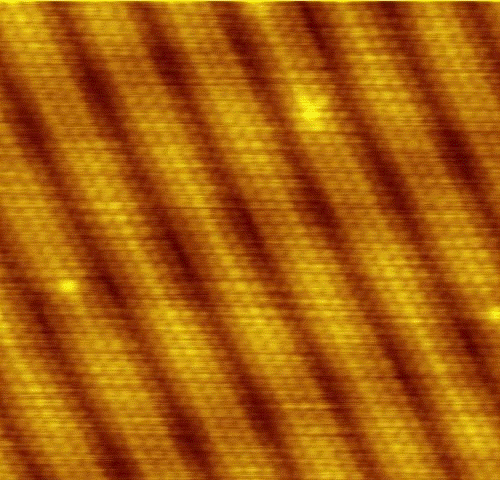
Individual atoms of gold can be seen on a clean surface by using a scanning tunneling microscope.

A crystal of cubic zirconia looks nearly identical to diamond yet is made of different atoms.
Some materials are made of only one element and others are mixed together to make compounds. For example, a perfect diamond is made of pure carbon in a 3 dimensional crystalline arrangement called a diamond crystal structure. Cubic zirconia looks very similar to diamond but is chemically very different than carbon; cubic zirconia is made of atoms of zirconia (Zr) bonded with atoms of oxygen (O).
Even with the same exact chemical components, a totally different material can be made just by changing the structure. Graphite, like diamond, is pure carbon. Instead of being a diamond crystal, however, in graphite the carbon atoms are bonded in a flat hexagonal arrangement. Just this one change to the bonding structure makes graphite totally different than diamond. Graphite’s appearance, mechanical, electrical, and thermal properties are very different than that of diamond. Graphite is used to make pencil lead (the graphite in lead is mixed with clay).

Graphite is chemically identical to diamond; both are pure carbon, but the electrons are shared differently between carbon atoms.
Other materials can have different properties by changing the concentrations of the atoms in the material, like different grades of carbon steel.
The interesting thing is that the properties of matter, including the electrical properties of materials used in electronics, all begin with the atoms of the materials themselves. In order to understand how this works, we need to start with the atoms.
Ancient Atomic Theory
The idea that all matter is composed of atoms dates back to the ancient Greeks, but similar ideas were hypothesized by many different cultures. However, these theories remained in the realm of philosophy for many centuries.
At the same time, many cultures hypothesized the existence of elements that were sometimes linked to theories about atoms. The four classical elements were earth, air, fire , and water with similar lists of elements developed in many ancient cultures such as Greece, India, Persia, China, Egypt, Japan, Tibet.
In the Medieval era, alchemists played an early role in uncovering different chemical elements, and used the term ‘element’ to describe them, hearkening back to the ancient four elements of earth, air, fire, and water.
What came to be known as atomic theory emerged with the work of chemists who had discovered that various chemical phenomena could be explained by hypothesizing that chemicals were made of different elements.
Dalton’s Atomic Theory
In the early 1800’s, the English schoolteacher John Dalton was the first to connect the idea of chemical elements to that of unique atoms.
Dalton hypothesized that:
(1) Every element is composed of small particles called atoms.
(2) Atoms of a given element are identical but different from atoms of all other elements.
(3) Atoms can’t be changed into a different kind of element; they can’t be created or destroyed by chemical reactions.
(4) Compounds are formed from different combinations of atoms.
(5) Chemical reactions combine, separate, or rearrange atoms.
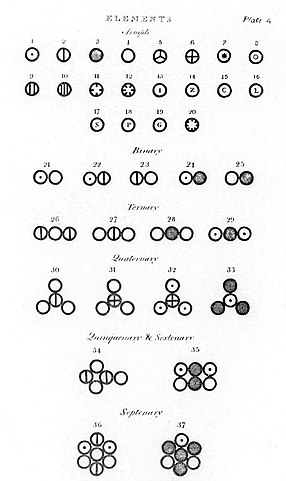
Illustrations of atoms and molecules in Dalton’s A New System of Chemical Philosophy.
The incredible thing about Dalton’s hypothesis is that he had no direct knowledge of atoms themselves. It wasn’t until scientists began experimenting with cathode-ray tubes in the mid-1800s that we began to explore atoms and atomic structure in more detail.
The Structure of an Atom
Cathode Rays
Interestingly, the study of atomic structure began with an electrical experiment.

Fluorescence in a neon lamp is caused by cathode rays (electrons).
Scientists found that applying a high voltage to a partially evacuated tube (vacuum tube), would result in a then-unknown form of radiation. The radiation was called a cathode ray because it seemed to come from the tube’s negative electrode, or cathode. Cathode rays couldn’t be directly seen, but they would cause any gas in the tube to produce light, or fluoresce, like a modern neon light.
Many experiments were done, and different theories were produced regarding what this mysterious radiation actually was.
As interesting (or scary) sounding the term ‘cathode ray’ was, it took decades to figure out what cathode rays actually were.
Plum Pudding Model
In 1897, JJ Thomson published a paper concluding that cathode rays were made of a stream of negatively charged particles. These particles would later become known as electrons.
Thomson was able to estimate the mass of the electron, realizing that electrons are far smaller than atoms themselves. This discovery led him to believe that electrons are only a small fraction of atoms.
He proposed a theory that atoms were positively charged spheres into which electrons were embedded.
This theory became known as the plum pudding model.
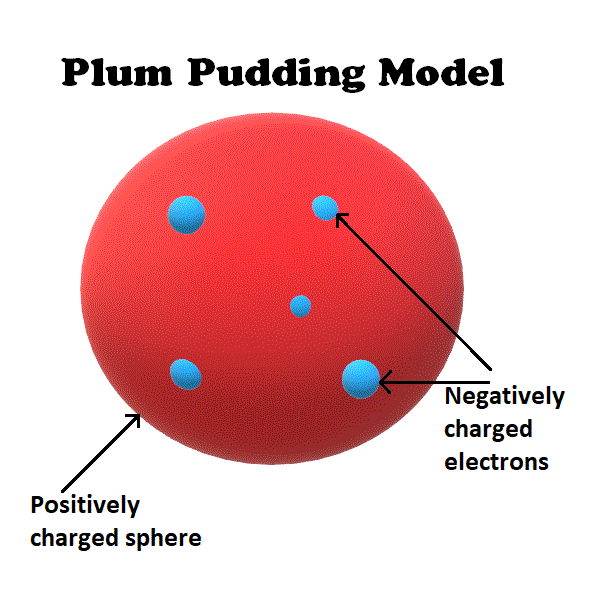
Although the plum pudding model proved to be incorrect, it was a useful stepping stone toward a better atomic theory.
Rutherford Model
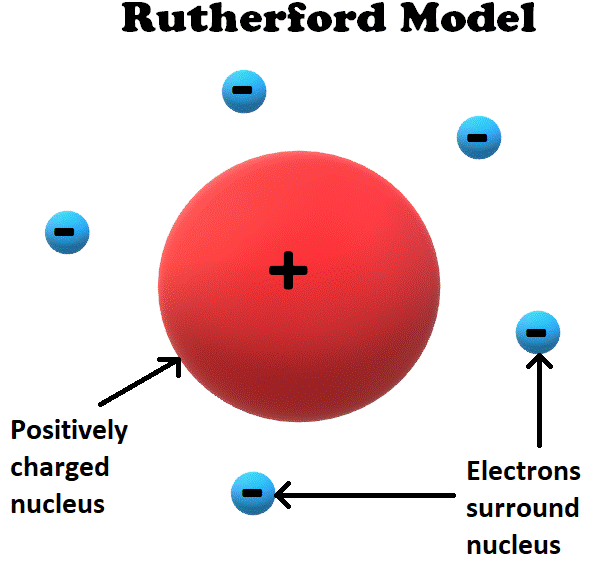
In 1911, Thomson’s student Ernest Rutherford realized that the plum pudding model was inconsistent with his own experimentation.
Based on his results, Rutherford proposed a new theory in which the positive charge was located in a dense location in the middle of the atom, called the nucleus.
In Rutherford’s model, the positively charged nucleus held most of the mass of the atom and was surrounded by a ‘cloud’ of negatively charged electrons.
This model was significant not only because it was ‘more correct’ than the plum pudding model. It was also the first theory in which atoms contain their own independent particles that are smaller than itself.
Bohr Model
Two few years later, in 1913, Rutherford and colleague Niels Bohr presented the model which is still used to teach atomic theory today.
This model, called the Bohr, or Rutherford Bohr model, is similar to the Rutherford model. The primary difference is that the electrons orbit the nucleus similar to the way planets orbit the sun in the Solar System.
Electrons are confined to specific orbits that are called atomic shells; electrons can’t just orbit the nucleus freely but can only orbit at specific distances from the nucleus.
Each atomic shell can also only hold a certain number of electrons; larger atoms require more shells that are farther from the nucleus.
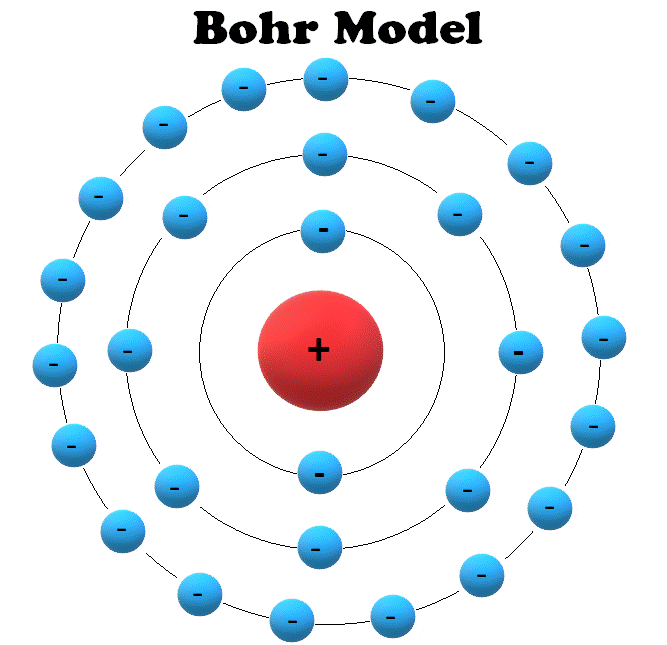
In this illustration of the Bohr model, each circle surrounding the nucleus represents an atomic shell. Each shell can only be occupied by a specific number of electrons.
Limitations of the Bohr Model
The Bohr model is an excellent introduction to atomic theory but it still has limitations. It’s description of electrons is very helpful but not completely accurate. It didn’t include protons or neutrons, but protons were soon embraced and actually named by Rutherford in 1920. Rutherford also proposed something akin to the existence of neutrons in 1920, but he believed that neutrons were a combination of protons and neutrons bound tightly together in the nucleus.
It also took time for scientists to figure out why protons in the nucleus stick together instead of repelling each other due to electrostatic repulsion.
Modern Atomic Theory
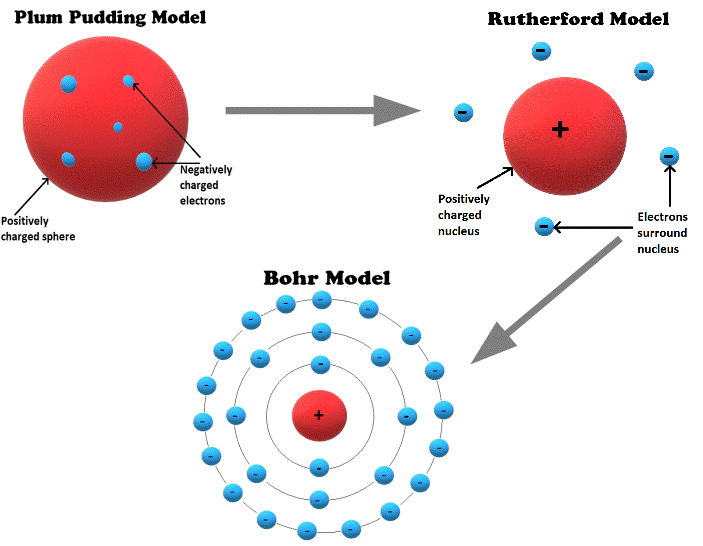
We have seen that atomic theory progressed from the earliest observations of cathode rays to the plum pudding, Rutherford, and Bohr models of the atom. The Bohr model is still commonly taught today as the basis for modern atomic theory.
In modern atomic theory, an atom is a group of particles that is joined together by two different forces.
In the middle of every atom is its nucleus. The nucleus is a tight core made up of two different particles, neutrons and protons. Neutrons and protons are held together with something called the strong force or strong nuclear force, thereby creating the nucleus.
Protons hold electrons in orbits that surround the nucleus. The attraction between protons and electrons is described by electric charge, and the force due to electric charge is called the electromagnetic force.
So, protons and neutrons are held together by the strong force, and protons and electrons are held together by the electromagnetic force.
The three particles that make up atoms also have different properties.
Neutrons
A neutron is a subatomic particle that has mass but has no electric charge. Since they have no electric charge, we can say that they are neutral.
Protons
A proton is a subatomic particle that has about the same mass as a neutron, but has a positive electric charge. The number of protons determines the element of the atom.
Electrons
An electron is a much smaller particle than neutrons or protons; electrons have about 1/2000 the mass of a proton. Yet its electric charge is exactly equal and opposite to that of the proton.
Electrons can only occupy specific orbitals, with specific energy levels and other characteristics. One major difference between modern atomic theory and the Bohr model is that electrons no longer ‘orbit’ the nucleus. Instead, quantum mechanics tells us that electrons exist as waves that can take on really weird shapes.
Each electron orbital can only hold two electrons.
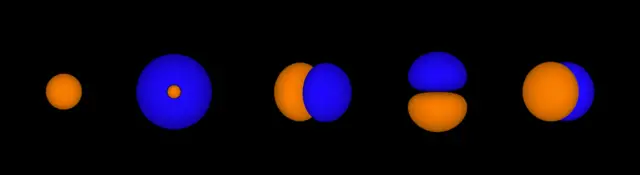
The first five electron orbitals. Each orbital contains two electrons.
Isotopes
Atoms of the same element may have more or less neutrons, and the different atoms are referred to as isotopes.
Ions
Similarly, atoms of the same element may have more or less electrons, and an atom with a net electric charge (i.e. not neutral) is referred to as an ion.
Valence Electrons
The electrons closest to the nucleus are very tightly bound to it, since the total positive charge of the nucleus is so much greater than the electrons close to it.
The farther the electron is from the nucleus, the less powerful the attraction from the nucleus is.
The electrons that are the farthest from the nucleus are called valence electrons. Valence electrons are the most weakly bound since they are far from the nucleus, and the ‘pull’ from the nucleus is screened, or partly blocked, by the inner, non-valence electrons electrons that are between the valence electrons and the nucleus.
Valence electrons are the most weakly bound electrons in any atom, but their actual bond depends on the material itself. In some materials, like the noble gasses, valence electrons are still tightly bound enough that the atoms remain inert. However in other materials, like conductors, valence electrons are so weakly bound that only a small amount of energy is needed to free them from their atoms.
Electricity is therefore generally a product of the valence electrons. The valence electrons, especially in good electrical conductors, are so weakly bound to the nucleus that they are almost unattached; a very small applied voltage has enough force to separate the valence electrons from the atom and allow the valence electrons to move- generating electric current.
Valence electrons are also important for chemical bonding. Atoms want to complete their outermost shell, and will share or transfer electrons with other atoms to achieve this.
We’ll cover many of these topics in greater detail later in the course, including the quantum mechanics behind the atom- somewhat surprisingly, the same information from quantum mechanics to understand how electrons exist in orbitals, is also needed to understand the physics of semiconductor devices like diodes, transistors, and lasers.
Properties of Atomic Particles
Electric charge is a fundamental property of many subatomic particles. What does this mean? At the most basic level, scientists have found that the particles that make up the entire universe have three distinct properties that define how they interact with everything in the universe itself.
These three properties are mass, spin, and electric charge. Not all particles have all three properties, but all particles have at least one.
| Subatomic Particle | Mass (MeV/(c^2)) | Spin | Charge |
| Proton | 938.28 | 1/2 | +1.602 x 10-19 C |
| Neutron | 939.57 | 1/2 | 0 |
| Electron | 0.511 | 1/2 | -1.602 x 10-19 C |
to that of protons.
Mass
Mass is defined by the resistance of any object (or particle) to acceleration. In other words, how much force does it take to move the object? Mass is mathematically described by Newton’s Second Law:
F = ma \\ ... \\m = \frac{F}{a}The property of mass also defines the gravitational attraction of objects. The more massive an object is, the more it attracts every other object with mass – in the whole universe.
Mass is defined by the resistance of any object (or particle) to acceleration. In other words, how much force does it take to move the object? It also determines the strength of gravitational interaction; the more massive the object, the more it attracts objects around it via gravity.
Not all particles have mass; the photon is probably the most commonly known particle that does not have mass.
As you can see in the table above, the mass of an electron is much less than the mass of either a proton or neutron.
Spin
Spin is a property that is similar to the angular momentum of a spinning object; while subatomic particles do not physically rotate, some of them act as if they do. It may seem confusing, but this topic does require some knowledge of quantum mechanics to understand- so don’t feel bad!
In 1922, Walter Gerlach performed an experiment with silver atoms. He used a furnace to create a beam of silver atoms that he directed with two magnets, and recorded where the silver atoms landed on a target. He expected to see a continuous distribution of atoms. Instead, he found that they created two piles; one above the beam and one below it, which nothing in between (which should have been the area of greatest concentration). This experiment indicated that there was some quantized angular momentum in the atoms themselves. The word ‘quantized’ just means that instead of being in a range of values, only a few values ware possible. In this case, Gerlach knew that whatever the angular momentum of the silver atoms was, it could only occur in two possible values, one positive and the other negative, which was why the atoms formed two piles.
We learned earlier that each atomic orbital can contain two electrons. In other words, each orbital contains two ‘states’ that can be occupied by electrons. Each state corresponds to a spin value of the electron occupying the state.
The two states in electron orbitals are called ‘spin-up’ and ‘spin-down’ states.
It turns out that spin is the origin of magnetism. When an orbital contains only one electron, it is called an unpaired electron. In most materials, the electrons are arranged with the spin states pointed randomly. If the spin states of unpaired electrons are in the same direction, the material becomes magnetic. Iron is magnetized because it has two unpaired electrons and their spin states tend to align with each other. This is called ferromagnetism.
Electric Charge
Electric charge is the property that causes electrons to repel other electrons and to attract protons, which creates atoms. It also allows us to control electrons, which is how we generate electricity and make electronics work.
Electric charge is defined as the property that causes a particle or object to experience a force when placed in an electromagnetic field. A neutron will not move if you place it in an electromagnetic field, but a proton or electron will- because protons and electrons each have an electric charge.
The most fundamental concepts about charge are:
Positive charges repel other positive charges and attract negative charges.
Negative charges repel other negative charges and attract positive charges.
You probably already knew this, but you might not have known just how important it is. Together with other laws governing the structure of atoms, the principles of charge attraction and repulsion explain why atoms form from subatomic particles instead of the universe just being a weird particle soup.
Advanced Physics: The Four Fundamental Forces
In physics, every single interaction that we have discovered (thus far) can be boiled down to one of four fundamental forces. These are (1) gravitation, (2) electromagnetism, (3) the strong nuclear force, and (4) the weak nuclear force.
The contents of this website are dedicated to understanding electromagnetism as it applies to engineered systems. However, it’s useful to have some understanding of these forces in general. For instance, earlier in this article we discussed the fact that protons and neutrons are bonded by the strong nuclear force. You don’t need to know much else about the strong nuclear force, but a few general principles will greatly help your comprehension of how the universe works, and with it, electronics too!
Gravitation: Gravity is the attraction between all things with mass. Gravity is the weakest of the forces. The only reason we stick to the earth’s surface is that the earth is so massive. Even a small quantity (the size of a basketball) of electrons would have a greater force than the earth’s gravity.
Electromagnetic: The electromagnetic force, as we’ve seen, is the interaction between electrically charged particles. Protons and electrons are by far the most common, but there are lots of other particles in the universe, too. Some of them are charged, like protons and neutrons, and some of them are not charged, like neutrons. The electromagnetic force is the 2nd strongest force. Not only is electromagnetism responsible for all electricity and electronics, it also keeps atoms together by the attraction between protons and electrons. The electromagnetic force is also responsible for magnetism and light.
Strong Nuclear Force: The force between protons and neutrons in the nucleus. Actually, the strong nuclear force doesn’t only hold protons and neutrons together in the nucleus. Both protons and neutrons are made up of combinations of even smaller particles called quarks. A proton contains two ‘up’ and one ‘down’ quark. A neutron contains two ‘down’ and one ‘up’ quark. The strong nuclear force is what holds these quarks together, creating protons and neutrons. It does double duty holding the protons and neutrons together to make the nucleus of an atom.
Weak Nuclear Force: The weak nuclear force is responsible for radioactive decay. It’s really weak compared to the strong force and electromagnetic force but still stronger than gravity.
We’ll continue to explore the relationship between atoms, electrons, and electricity in:
Lesson 5: Electrical Properties of Materials