What is Electricity?
Electricity is a natural phenomenon that is harnessed by humans in order to do useful things. Despite being complex, electricity is relatively simple to define. Electricity is a type of energy that results from the accumulation or flow of electric charge.
Electric charge is a fundamental physical property, and all electricity originates from the charge of protons and electrons in atoms.
There are many phenomena associated with electricity, but it can essentially be divided into two types. Static electricity is the due to the accumulation and separation of electric charge. Current electricity is the flow of electric charge.

Fig 2: Solar panels provide a sustainable source of electricity.
Despite being an essential and necessary part of modern civilization, electricity is a complex subject that can be difficult to understand. Electricity can often seem like magic.
And that’s not a bad thing! Without electricity, we would have no lights, no computers or cell phones, or most of the other technologies that make modern life great. In fact, almost everything we use or create relies on electricity. Our water and sewer systems, the farms that produce our food, the hospitals we are treated in when we get sick, and the factories that make virtually every product in the world today are all powered by electricity.
Electricity and Electric Charge
Electricity is usually defined as a complex set of physical phenomena (things that happen) that all relate to charged particles.
When we say ‘charged particles’, we are talking about the two types of charged particles in any atom: protons and electrons.
Electric Charge in Atoms
Within every atom, there are three types of particles; protons, neutrons, and electrons.
Protons and electrons have an electric charge that is equal and opposite. This is why electrons repel electrons, and protons repel protons, but protons and electrons attract one another. Neutrons don’t have an electric charge.
Protons are located at the center of every atom, which is called the nucleus. They have a positive (+) charge and stick tightly to neutrons. Together, protons and neutrons make up the nucleus of an atom.
The electrons surround the nucleus of the atom and form a type of shield around it. This means that protons and neutrons don’t interact much with other atoms.
Electrons are on the outside of an atom. Electrons ‘orbit’ the nucleus and have a negative (-) charge. Electrons don’t actually orbit the nucleus the way that planets orbit the sun, but it is a useful way to think about them.
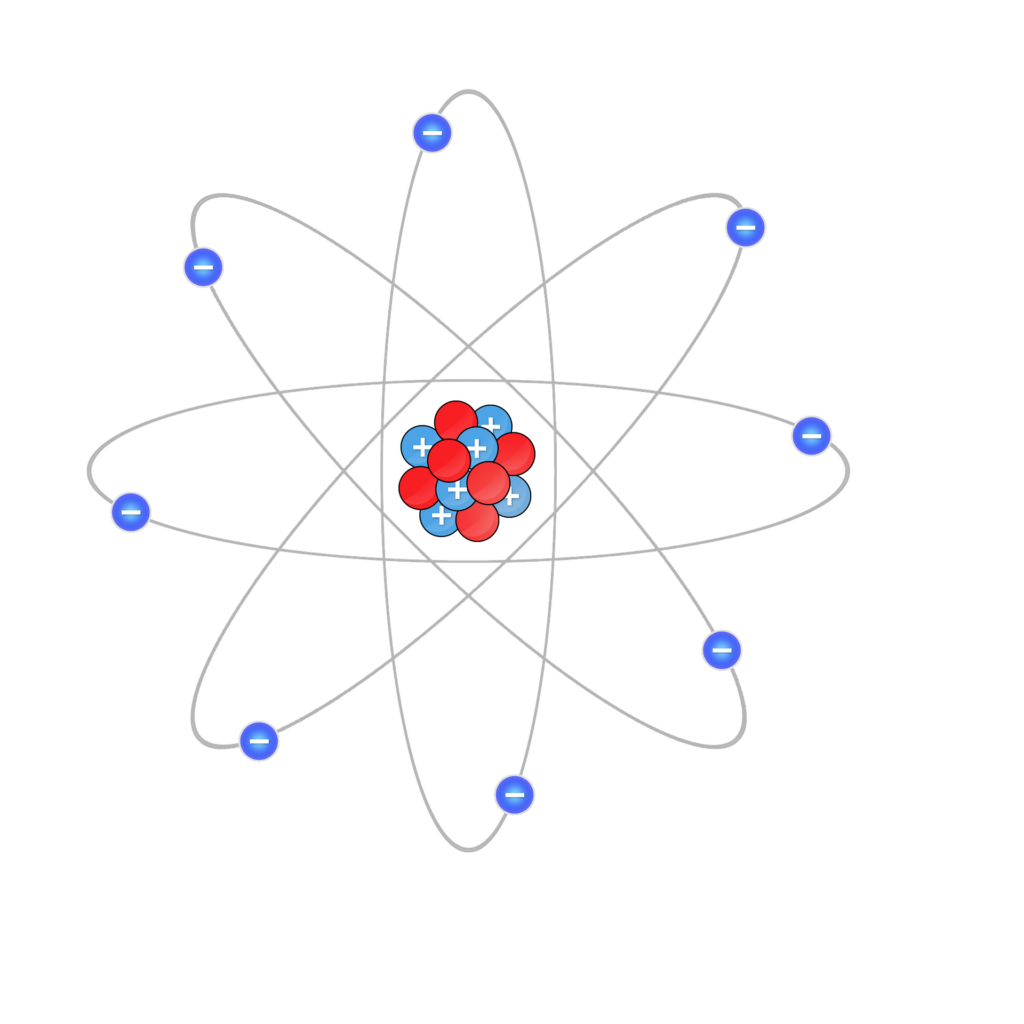
Electrons are the negatively charged particles that surround the nucleus in an atom.
The Importance of Electrons
Since electrons are on the outside of the atom, they do almost all of the interacting for the atom itself.
Chemical bonds are formed when electrons are shared or exchanged between atoms.
Electricity occurs when electrons are separated from their atoms. It doesn’t take much energy to separate an electron from an atom; just walking on a carpet is enough to separate enough electrons from their atoms to make a spark.
This is why electrons are super important for electronics and electricity in general.
Did you know? There’s so much more to the atom than protons and electrons.
Electrons are considered to be ‘elementary particles’, meaning that they don’t have any sub-particles. But protons and neutrons are made of quarks. Protons have two ‘up quarks’ and one ‘down quark’, bound together with gluons.
Positive and Negative Electric Charge
The choice of the word ‘negative‘ instead of ‘positive‘ for the charge of the electron wasn’t intentional. It was chosen because scientists learned about charge after they had learned about electricity.
You can blame Benjamin Franklin. He had to pick one, but our lives might be a little easier if he chose ‘positive’ to describe the electron.
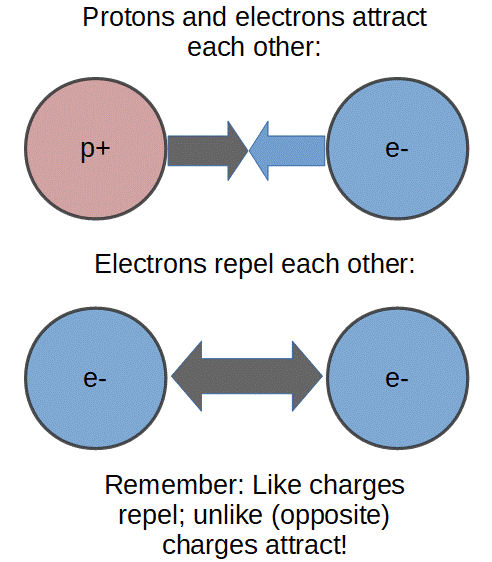
It turns out that it doesn’t really matter what we call positive and negative charge, but now that it’s standard, we do have to remember which is positive (proton) and which is negative (electron).
Perhaps the most important thing to remember is the adage:
Like charges repel and unlike (opposite) charges attract.
In fact, the quality of attraction and repulsion is exactly what we call ‘electric charge’.
The attraction between electrons and protons is what holds an atom together. This attraction, called the electrostatic force, must be overcome for electricity (i.e. electrical activity) to occur.
How Atoms Lose Their Electrons
In order for electrical activity to occur, an atom must lose an electron. This happens by giving the electron enough energy to escape from the attractive force of the protons in the atom.
Rub a finger on an object. You might be able to rub some of the electrons off the surface, which is exactly what happens with static electricity. In this case, the mechanical energy from your finger rubbing against the object provided enough energy for some of the electrons in the object to escape.
Any kind of energy can separate an electron from its atom. Chemical energy from a chemical reaction commonly separates electrons from atoms. Energy from light shining on an object can also separate electrons from their atoms; this is called the photoelectric effect. The photoelectric effect is how solar cells are able to generate electricity from sunlight.
Solar panels use energy from sunlight to produce electricity.
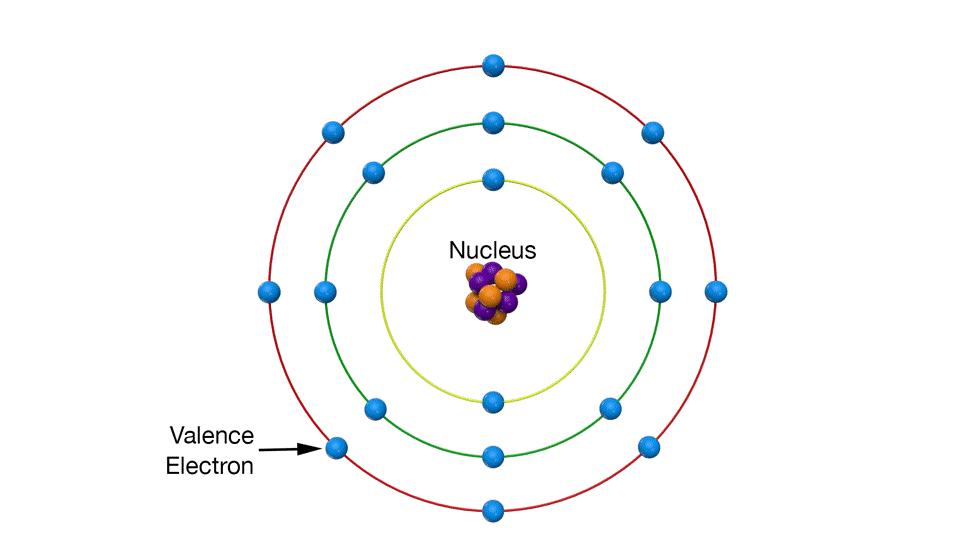
Valence electrons are in the outermost electron ‘shell’.
In general, the electrons that do the interacting are the outermost electrons, called valence electrons.
Valence electrons are the farthest from the nucleus so it takes the least amount of energy to separate a valence electron from its atom. Whenever you remove one electron from an atom, it takes much more energy to remove a second electron from the same atom. Most of the time, atoms only contribute one electron to electrical activity.
In addition, some atoms hold on to their electrons more tightly than others. Some electrons take a huge amount of energy to remove from their atom, and others don’t take much at all. It depends on the type of atom and also how that atom is chemically bonded to other atoms.
These atomic properties translate into material properties when we are dealing with normal-scale objects like wires in a circuit.
Materials Have Unique Electrical Properties
The universe is made of many different types of matter, and they are all unique. A type of matter is referred to as a ‘material’, like wood or gold or even air.
Every type of material has electrical properties associated with it. Materials are often classified electrically as conductors, insulators, or semiconductors.
Conductors
Conductors (like metals) have electrons that are really easy to remove from their atoms. The name conductor was chosen because their electrons move easily and electricity easily passes through them.
Conductors are used to transmit electricity and connect components in electric circuits.

Electrical wire consists of a conductor that is sheathed with an insulator to protect people and property.
Insulators

An industrial insulator for a power line.
Insulators hold tightly onto their electrons. The name insulator was chosen because these materials prevent electron flow, i.e. they insulate against it. However, given enough energy, anything can conduct electricity- including air or even a vacuum like in outer space!
Insulators are used to protect people and property from the dangers of electricity. Wires are often covered in an insulating sheath, and heavier duty insulators (like the example on the left) are used in many industrial applications.
Semiconductors
Semiconductors are materials that conduct electricity, but not as well as conductors. It might seem strange that semiconductors are useful. After all, they don’t conduct as well as a conductor, and they don’t insulate as well as an insulator.
Yet somehow, semiconductors have become the most important materials in the field of electronics! How is this possible?
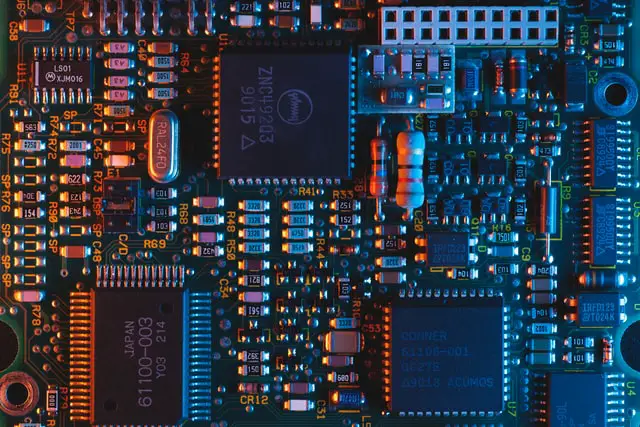
Modern electronics rely on semiconductor materials. The large blocks, known as ‘integrated circuits’, are all made from circuits that are created on a block of semiconductor material.
It turns out that semiconductors have electrical properties that can be modified relatively easily. This is accomplished with semiconductor doping.
In other words, the electrical properties can be changed by engineers in order to make some really cool things. Semiconductor components like diodes and transistors are used in integrated circuits to make things like television and computers possible.
The Two Types of Electricity
We have learned that if an electron is given enough energy, it can escape from an atom.
So, how does electricity actually happen?
The answer is that there are essentially two interrelated types of electricity that occur.
Once an electron is separated from an atom, it leaves behind a positively charged atom called an ion. This separation of charge is known as static electricity.
The charged particles might remain where they are, but they might also move if something, like another charged particle, attracts or repels them. The flow of electric charge is called electric current, and this type of electricity is called current electricity.
Static Electricity
Static electricity arises naturally due to the separation of charge whenever an electron leaves an atom. If enough charge builds up, a spark – or discharge – may occur.

A woman’s hair stands on end due to static electricity, as she touches a Van de Graaf generator.
As soon as an electron is freed from an atom, a separation of positive and negative charge has occurred.
This separation of charge is described by the term static electricity.
Just like the attraction between electrons and protons, there is an attraction between positive and negative charges.
They exhibit a force on each other.
That force is important to all forms of electricity, natural and man-made. The scientific term for it is called the electrostatic force.
If you rub an object and take it’s electrons, your body becomes negatively charged (usually just a little). The object that you rubbed now has less electrons than it started with. Since electrons are negatively charged, your body is a little negatively charged. The object has lost some electrons, so it is now positively charged.
Studying the history of electricity is a great way of learning about it!
People have known about and experimented with static electricity since at least Ancient Greece. In the 5th century BC, Thales of Miletus noticed that leaves would stick to amber that he rubbed. He also noted that two pieces of rubbed amber would repel each other.
Current Electricity
Current electricity is another form of electricity that we all experience in daily life. It is the kind of electricity that powers our societies.
When you rub a suitable object or walk across a carpet, you can build up a good amount of charge in your body. If you then touch a metal object, you might feel a snap or even see a spark of static electricity. That’s called static shock or electrostatic discharge, as shown in the image above.
Static shock occurs because the electrons repel each other and were given a conductive path to flow through. This movement of charged particles through a conductive path is called current electricity. Current describes the flow of electric charge through a system. The ultimate ‘goal’ of conducting electrons is to re-join an atom.
When lightning strikes or you are shocked by static electricity, it is because the electrons influence the atoms around them. They want to travel to a place where they can join atoms with a positive charge and make neutral atoms again.
The electrostatic force is responsible for all electricity because a charged object influences all of the atoms around it. When we use electric circuits, we set up a situation where electrons want to flow through the circuit due to this force.
We do this by using a voltage source.
Electrons can be made to move from one place to another because of a difference in electric potential, also known as voltage. Voltage is like the electric equivalent of gravity; when electrons are connected from a point of high potential to a point of low potential, they will spontaneously flow. The force causing the electrons to move is the electrostatic force. When the electrons move, the electron flow is called electric current. That’s exactly how electric circuits work.
Let’s look at a couple of familiar examples of naturally occurring electricity to help us develop an understanding of what electricity really is.
Electricity in Nature
Static Shock
Let’s return to the example of static electricity.
Even if the points aren’t physically connected, if there are enough electrons built up, they may jump through the air. The object charged with extra electrons discharges them across the air, creating a spark.

How does this actually happen?
When you walk around, your clothes rub against your skin and other materials. Your body builds up electrons, which results in a build-up of electrical charge (one electron worth of charge for each electron).
Every electron has the same exact charge, and there is no way for an electron to have more or less charge. The negative charge that you accumulate repels other electrons in objects around you. Most objects (like your couch, or kitchen table) are electric insulators. When you get close to them, the repulsion between the extra electrons in your body and the electrons in the table or couch won’t do much.
When you get close to a metal object, though, the negative charge in your hand causes the electrons in the metal to be repelled by your hand. Since a metal is a conductor, some of its’ electrons actually move away from their atoms. So when you touch a metal, the atoms that you touch are actually positively charged. If you have enough charge in your body, you don’t even need to make contact with the metal for electric current to flow. When the negative charge in your hand gets close to the positively charged atoms in the metal, ZAP!!! The electrons JUMP through the air, creating a spark.
Note that even this small amount of current can be painful which is why it’s so important to be safe when working with electricity.
Thunderstorms

Let’s look at another example to help show the fundamental nature of electricity: the thunderstorm.
In a thunderstorm, the water in the cloud can be very cold because it’s high up in the sky, about -15 to -25 Celsius. Even if the temperature on the ground is warm, the cloud is much colder. The center of the cloud is made up of ice crystals, hail, and snow.
The air moves really fast in a thunderstorm. An updraft carries small droplets of frozen water (ice) to the top of the cloud. Larger bits of snow and ice are heavier and fall to the bottom of the cloud. This movement in the cloud causes the atoms to collide, push and roll against each other with a lot of force.
The forces between the atoms as they rub against each other can give the electrons in the atoms enough energy to escape from the atom.
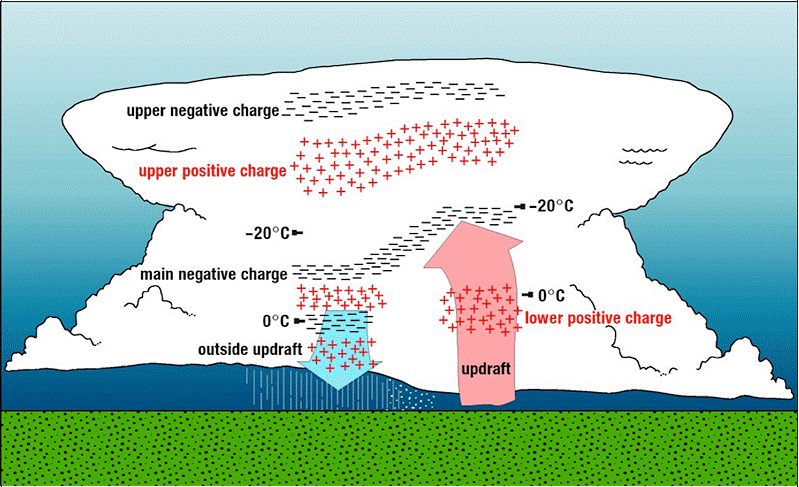
The internal structure of a thunderstorm. Lightning strikes when enough charge builds up.
The top of the cloud becomes positively charged with atoms that have lost some electrons. The bottom of the cloud becomes negatively charged with atoms that have gained electrons. These cause other positive and negative regions to form in the cloud, as positively charged ions are attracted to negatively charged ions and vice versa.
With enough charge separation, electricity can flow through the air. It takes a big difference in electrical potential for electricity to conduct through the air, because there is a large distance from the cloud to the ground. Lightning strikes with a huge amount of power.
Man-Made Electricity
Static electricity and lightning are great ways to introduce some of the important concepts related to electricity. In our homes, schools, and workplaces, we use electricity that is intentionally generated, transmitted, and used in specific ways. Electricity is typically generated at power plants, and is then transmitted to our homes and businesses so that we can use it.
Understanding this man-made type of electricity is the focus of this course.
Electric Circuits
Man-made electricity is used in electric circuits. From the point of generation to the final usage, the electricity flows through different circuits that each play a role in making the electricity as useful as possible.
Circuits are paths for electricity to flow in that allow us to engineer specific properties and functions. They allow us to do useful things with electricity.
Circuits contain conductors that form a path for electrons, and components like resistors, capacitors, and transistors to create a system that does something useful with electricity. We’ll learn about these components and many different examples of circuits in this course.
Let’s conclude this lesson with a familiar example: the humble light bulb.
Light bulbs
If you’ve ever turned on a light in your house, you know how to use a switch to close and energize a circuit. The light switch is a device that is identical in function to any other electrical switch. A switch allows you to connect and disconnect the two sides of a circuit- the positive and negative terminals.
When you turn a light ON, you close the circuit and enable current to flow from the positive side, through the lightbulb, to the negative side. When you turn the light OFF, you disconnect the two sides of the circuit from each other. There is nowhere for the current to flow.

This is called opening the circuit- current cannot flow until you close the circuit by flipping the switch again. When the light is OFF, electrons are patiently waiting in the lightbulb’s filament for you to reconnect them to the circuit. The electrons can’t move, so there is no electric current flowing through the lightbulb.
It’s not the electrons themselves but the MOVEMENT of electrons through the lightbulb that causes it to light up.
You can think of the electrons like cars sitting in traffic. When the road is clear, cars will move and traffic will flow. When you block the road, the cars cannot move anymore. Instead, they need to wait for the car in front of them to move; the traffic has stopped. Unlike cars, all of the electrons in a circuit all move at about the same exact time. In traffic there is always a delay as each person waits for the car in front of them to move before moving themselves.
You’ve come a long way toward a thorough understanding of electricity. It’s time to move on to the next topic:
Lesson 2: History of Electricity