Electric Charge
Electric charge is the property of matter that causes any matter (objects or particles) with charge to experience a force exerted by other charged matter.
Electric charge is one of the most important concepts in physics, chemistry, and electrical engineering. It is responsible for the structure of atoms and of bulk materials. Electric charge both holds atoms together and also prevents atoms from entering each others’ spaces.
You have probably never thought to ask why you don’t sink into the floor when you walk, or why you can use an object without your hand going through it, but the answer is electric charge. The electrons in your body repels the electrons in the objects that you touch.

Electric charge is fundamental to every aspect of electricity and electronics. By understanding electric charge, we can continue to build on our understanding of electricity and begin to explore the fundamental interactions that will allow us to learn about electrical current at a deeper level.
The Property of Electric Charge
The main action of electric charge is that like-charged objects repel (called electrostatic repulsion) and opposite-charged objects attract (called electrostatic attraction).
Charge can either be positive or negative. When we say that two objects are ‘like-charged’, we mean that the net charge of the two objects have the same sign; either they are both negatively charged or both positively charged. When we say that two objects are ‘opposite-charged’, we mean that one object has a net positive charge and the other object has a net negative charge.
Electrostatic Repulsion
Two electrons (or protons) have the same charge, so they repel each other:
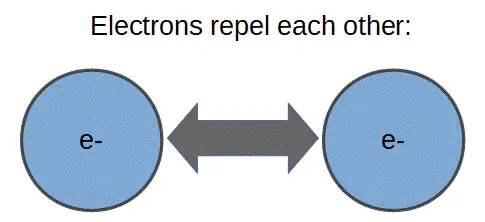
This is called electrostatic repulsion. It occurs with all ‘like charged’ objects. For instance, two ions with negative charge (i.e. atoms that have gained an electron) will repel each other. The same will happen for two ions with positive charge.
Electrostatic Attraction
Opposites attract.
Protons and electrons have the opposite charge, so they attract each other.
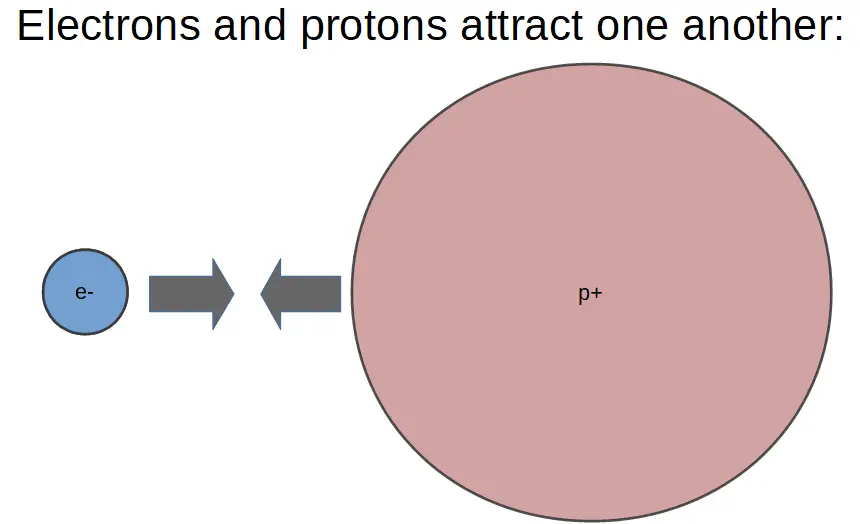
This is called electrostatic attraction. It occurs between any two objects that are oppositely charged. An ion with a positive charge will be attracted to an ion with a negative charge.
Protons have a charge of (positive) + 1.602 x 10-19 coulomb, and electrons have a charge of (negative) -1.602 x 10-19 coulomb. The charges have the opposite sign, so they attract one another. A coulomb is just the SI unit for charge (i.e. how we measure and compare quantities of charge). In terms of physical size, protons are much bigger than electrons (i.e. protons have more mass than electrons), but the force exerted by each is the same. In other words, electrons have a much higher charge to mass ratio than protons.
The force pulling electrons and protons together depends on the charge of the particle, not the mass. However, because the mass of the proton is much greater, it will move less distance than the electron when experiencing the same force.
Electric Charge is Deceptively Strong
Electric charge exerts a deceptively strong force. The technical name for the attraction or repulsion between charged objects is called the electromagnetic force.
How strong is the electromagnetic force?
For comparison, the electromagnetic force is 1036 times stronger than gravity.
It’s actually really important that the force of electric is so strong, otherwise electrons could be pulled away from atoms by gravity, and atoms would collapse into each other because the repulsion between electrons would be too weak to prevent it.
If Electric Charge is So Strong, Why Does it Seem Weak?
The strength of the force exerted by electric charge seems weak because the typical atom is electrically neutral. The attraction between protons and electrons help the atom to stick together, and the repulsion between electrons does help prevent atoms from collapsing into each other.
But at a large distance away from an atom, the positive and negative charges within it balance out and exert a net zero force. So while there’s a low of charged particles, like protons and electrons in the earth, the net electromagnetic force on our bodies is zero. This is due to to the fact that charge can be either positive or negative, and they cancel each other out.
In contrast, gravity doesn’t have a ‘negative gravity’ counterpart to cancel out its’ effect. The gravity exerted by a single particle is very small, but it adds on to the gravity exerted by every other particle that it is near. So every atom within the earth contributes to the overall force of gravity, which feels really strong. Yet the electromagnetic force is still strong enough to allow us to walk around the earth’s surface without being pulled down into the center of the earth.
SI Unit of Electric Charge
The term SI stands for Système international, which is a worldwide system of standardized measurements. The SI system basically extends the metric system to lots of measurable properties like force, pressure, time, voltage, and electric charge. For reference, you can find a table of standard unit measurements here.
The SI unit for charge is the Coulomb, with an abbreviation of ‘C’. For example, a charge of 5 Coulombs is written as ‘5 C’.
An interesting aspect of electric charge is that because the charge of an electron (or proton) is an inherent property of particles in nature, it makes sense to define measurement units using this naturally occurring baseline.
Thus the unit of Coulomb is defined by the charge of a certain number of electrons. Originally, the Coulomb was defined as the amount of charge carried by 1 ampere of current in one second, which is a lot of electrons. This was sue to the fact that current was discovered before charge.
For this reason, the official exact definition of one Coulomb is the charge of 6, 241, 509, 074, 460, 762, 607.776 electrons.
Of course, a perfect Coulomb of charge is impossible to achieve because it includes a decimal of .776 elementary charges, and neither electrons nor protons can be split into a fraction.
However, this doesn’t stop the Coulomb from doing its’ job as a unit of measurement.
Elementary Charge: The Electric Charge of a Single Proton
Measured in the SI Unit of the Coulomb, a single proton has a charge of about 1.6 x 10-19 Coulomb. This is called an elementary charge, which is abbreviated by the letter ‘e‘:
e = 1.60217662 \times 10^{-19} CA single proton has a charge of e, and a single electron has a charge of –e.
Keep in mind, charge is not a unit that most humans are very familiar with. In terms of electricity, it is much easier for use to understand voltage and current. For instance, a single AA battery will discharge about 5000 Coulombs over its life, whereas a lightning bolt may only transmit a handful! Yet, a AA battery is only 1.5 volts whereas a lightning bolt can strike at 1 Billion volts. We understand that the lightning bolt is much more dangerous than the battery because it’s voltage and current are much greater than that produced by the battery. The total charge doesn’t seem to matter much, but it is important as it determines the battery’s capacity.
Charge is Quantized
Electric charge only occurs in integer multiples of e; fractional charges are never found.
This is called charge quantization.
Total Charge vs. Elementary Charge
When we work with charge in equations, we typically use the abbreviation ‘q‘. For example, the total charge of 5 electrons would be written as:
q = -5e
If we wanted to determine how many Coulombs this is, we would write the following:
q = -5e = -5(1.6\times 10^{-19} C) = 8 \times 10^{-19} CHow Electric Charge Relates to Electricity
Electric charge is central to all forms of electricity and all systems that use electricity. When we investigate different aspects of electricity or electric circuits, we can always understand them in terms of charge. Some of the most important properties are voltage and current.
Voltage is the amount of energy required to move an elementary charge between two points. It is measured in units of Joules (energy) per Coulomb (charge).
Current is the rate of flow of electric charge. It is measured in units of Coulombs (charge) per second (time). The more electric charge (i.e. electrons) moves per second, the higher the current.